Biogeochemistry of iron in the ocean. Ocean acidification and global warming effect in the interaction of iron with the organic matter.
The research of the QUIMA group focuses on the effect of pH, T, O2 and organic matter in the behaviour of the Fe(II)-Fe(III) redox system in the North Atlantic Ocean, in order to compare these studies with experiments undertaken in the lab and to develop a global kinetic model for iron in the context of ocean acidification and global warming.
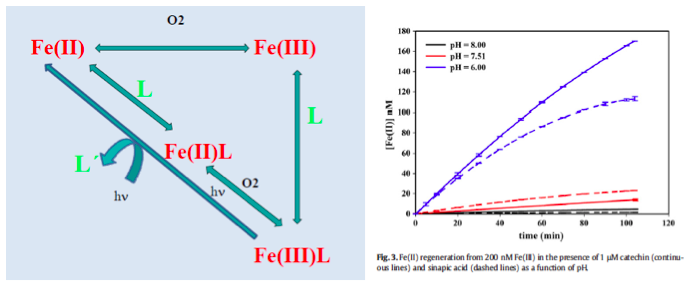
Iron has a high impact in the carbon cycle through its effects on planktonic communities and their productivity. Reduced iron, Fe(II), is the bioavailable species for eukaryotic phytoplankton, rather than oxidized iron, Fe(III). Fe(III) presents a low solubility and is mostly complexed by organic ligands. Fe(II) is thermodynamically unstable due to pH and O2 conditions in the medium and tends to become oxidised.
The acidification and the reduction of O2 favours the presence of Fe(II) in the medium. Moreover, organic matter plays an essential role in the Fe cycle in the ocean. The presence of organic compounds may reduce Fe(III) to Fe(II) and also stabilise Fe(II) by complexation. There is also an important competititon between iron and copper by the reactive oxygen species. It has been observed that in the presence of organic compounds like polyphenols or saccharides, the growth of eukaryotic organisms is favoured.
The main goals of this research is to investigate which compounds and mechanisms determine the presence of Fe(II) in the marine environment and how ocean acidification and global warming affect them.
To achieve these goals we combine studies of iron in the Atlantic Subarctic region, in the CLIVAR A1E (59.5ºN) hydrographic section and in the submarine volcano of El Hierro with studies in the lab using individual organic compounds and the exudates from cultures of phytoplankton. In the lab studies we consider the different variables independently in order to define the contribution of each one to the process.