Veronica Arnone (Ph.D. Student) "Organic complexation of Fe and Cu in marine environments"
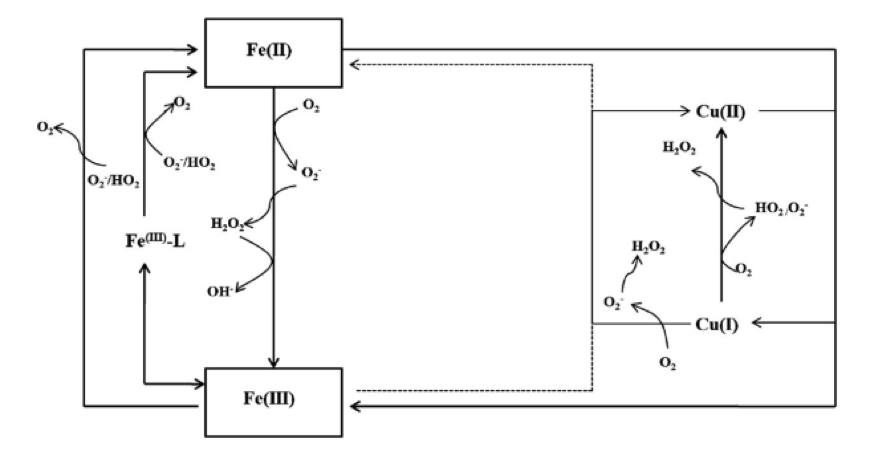
Iron (Fe) is an essential micronutrient for primary productivity in the oceans, serve as cofactors or part of cofactors in enzymes and as structural elements in proteins. Iron can be present in seawater as Fe(II) or Fe(III), its concentration fall below 0.2 nM in open ocean surface water and is controlled by redox reactions, inorganic and organic complexation, adsorption and precipitation processes. The organic complexation controls the bulk of iron speciation in seawater, varying its reactivity and bioavailability. However, the source and chemical structure of ligands remains mostly unknown. On the other hand, copper (Cu) is both a micronutrient and a growth inhibitor, due to its toxicity at high concentrations. This trace metal is part of proteins and enzymes but in recent years it has gained importance due to its relationship with the limitation of Fe. It has been discovered that iron and copper can compete for the same organic ligands and inorganic species (H2O2, O2•- and carbonate groups). Also, in response to Fe-limitation marine phytoplankton evolved physiological mechanisms to reduce their Fe requirements such as: using electron transfer proteins with alternative trace metal catalysts (Cu-containing plastocyanin instead of the Fe-containing cytochrome) and up-regulate a high-affinity Fe transport system containing a coupled ferrireductase and multiple-Cu containing ferroxidase. Therefore, the importance of these metals stems from the relationship between the ocean productivity and the carbon sequestration into the ocean. There is necessary to increase the knowledge about organic ligands, capable of complexing trace metals, and the interaction between different chemical species.